Abstract
The fall armyworm, Spodoptera frugiperda (J.E. Smith), is an invasive pest threatening crop production and food security worldwide. High concerns are linked to the potential establishment of the species in Europe. The high migratory capacity of S. frugiperda causes concerns about the potential impacts of transient populations invading new areas from suitable hotspots. In the present work, we developed and used a physiologically-based demographic model to quantitatively assess the risks of S. frugiperda in Europe. The risks were assessed considering a best-, a median-, and a worst-case scenario. The Mediterranean coastal areas of Southern Europe resulted particularly suitable for the establishment of the species, with suitable areas reaching even higher latitudes, in the worst-case scenario. In Europe, up to four generations per year were predicted. The predicted yearly average number of moths per trap per week (± standard deviation) was 5 (± 4), 17 (± 5), and 139 (± 22) in the best, median-, and worst-case assessment scenarios, respectively. Model results showed that Southern and Central Europe up to the 48th parallel north might be exposed to the risk of transient populations. Depending on the latitude and on the period of arrival of the propagule, 1–2 transient generations per year might be expected. The model can be used to define strategies for reducing the risks of establishment of the pest at the country level. Predictions on the dynamics and phenology of the pest can also be used to support its management at the local level.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Key message
-
There are high concerns linked to the potential establishment and impacts of Spodoptera frugiperda in Europe
-
We developed a physiologically-based model to quantitatively assess the risks linked to S. frugiperda
-
Risk of establishment of S. frugiperda was predicted in the Mediterranean coastal areas of Southern Europe
-
Risks linked to transient populations (i.e. populations migrating from suitable areas) were predicted in Southern and Central Europe
Introduction
The fall armyworm, Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae) is a phytophagous pest considered a major threat to agricultural production and food security (Early et al. 2018; Tambo et al. 2021), especially in developing countries (Devi 2018; FAO 2020; Suby et al. 2020; Koffi et al. 2020). The species is known to feed on more than 350 host plants including economically valuable crops such as maize, rice, soybean, sorghum, wheat, barley, and cotton (de Freitas Bueno et al. 2011; Hardke et al. 2015; Montezano et al. 2018). Impacts on crops are caused mainly by late instar larvae (Overton et al. 2021) feeding on stems, branches, leaves, and reproductive structures of the host, and causing direct yield loss, defoliation, and general weakness of the plant (Harrison 1984; Vilarinho et al. 2011). The larval trophic activity might favour plant infection caused by fungi (Farias et al. 2014). Yield loss to maize ranges from 11 to 67% (Hruska and Gould 1997; Day et al. 2017; Kumela et al. 2019; Baudron et al. 2019). Reported average losses for other crops are 26% for sorghum, 24% for sweet corn, 13% for bermudagrass, and 5% for rice. The control measures for protecting crops affected by the species and restrictions on the trade of potentially infested products cause further economic and social costs (Overton et al. 2021). The species is native to tropical and subtropical America where it is considered a prevalent pest for maize, soybean, cotton, and other major crops (Nagoshi et al. 2007; Baudron et al. 2019; Koffi et al. 2020). Human-mediated transportation and trades (Cock et al. 2017), the high migratory capacity (the species might fly up to 100 km per night) (Rose et al. 1975; Westbrook et al. 2016), and the high prolificacy (more than 1500 eggs laid per moth) (Luginbill, 1928) of the species facilitated the dispersal of the pest in non-native areas. In 2016, the species was accidentally introduced in Central and Western Africa (Goergen et al. 2016) where it was able to spread in vast areas of sub-Saharan and North Africa (Day et al. 2017; Cock et al. 2017; EPPO 2020a). Since 2018, the species invaded vast areas of the Middle East (EPPO 2019a; EPPO 2020b, c, d), South Asia (EPPO 2018; Sharanabasappa et al. 2019), South-Eastern Asia (EPPO 2019b, c; EPPO 2020e; Sartiami et al. 2020; Zaimi et al. 2021), East Asia (EPPO 2019d; Suh et al. 2021), North-Eastern Asia (EPPO 2019e) and Oceania (EPPO 2020f). In Europe, the species is currently (FAO 2021) present in the Canary Islands (EPPO 2021). S. frugiperda is on the EPPO A2 list of quarantine pests, and on the European Commission list of priority pests (EU 2019) due to the risk of introduction, establishment, and consequences of this pest to Europe. Fresh plant products imported from Latin America represent the main pathway of entry of the species into the EU (EFSA PLH Panel et al. 2017; 2018a; EFSA et al. 2020). Another pathway of introduction is represented by the possibility of eggs and adults to entry as hitchhikers on international flights (Early et al. 2018). The high migratory ability of the species causes concerns about the potential impacts of transient populations moving from hotspots to new areas during the favourable season (EFSA PLH Panel et al. 2018a; Timilsena et al. 2022). A realistic threat is the introduction of individuals from North Africa to Europe due to natural or wind-mediated dispersal (Westbrook et al. 2016; Early et al. 2018).
Given the potential threats of S. frugiperda to European agriculture, it is fundamental to quantitatively estimate the risk of establishment and the potential impacts linked to the species. This information is fundamental for planning and implementing surveillance and inspections to reduce the likelihood of introduction and establishment of the pest in Europe (EFSA PLH Panel et al. 2018a; EFSA et al. 2020). So far, many species distribution models have been developed for predicting the potential habitat suitability for S. frugiperda (Ramirez-Cabral et al. 2017; Du Plessis et al. 2018; Early et al. 2018; Liu et al. 2020; Baloch et al. 2020; Fan et al. 2020; Zacarias 2020; Huang et al. 2020; Tepa-Yotto et al. 2021; Ramasamy et al. 2021). However, there is high uncertainty on the risks linked to the establishment of this pest in Europe. For instance, no risk of establishment but only risk linked to transient populations was predicted by Du Plessis et al. (2018). On the contrary, suitable areas in Southern Europe were identified by Early et al. (2018) and EFSA PLH Panel et al. (2018a). Other authors identified risks in areas of Central Europe (Zacarias 2020) or further north, up to Ireland (Liu et al. 2020; Ramasamy et al. 2021) and Southern Norway (Tepa-Yotto et al. 2021), although with low habitat suitability indices. This high uncertainty reflects the need to establish sound criteria and reliable models for obtaining a realistic assessment of the risk linked to a pest (Ponti et al. 2015).
In this work, we aimed at providing a solid and quantitative assessment of the risks linked to S. frugiperda in Europe through the application of a physiologically-based (i.e. mechanistic) modelling approach. This approach allows for the faithful description of important aspects of the biology of the species (Sparks 1979), such as the nonlinear responses to temperature and the influence of relevant abiotic drivers (density-dependent factors, mortality due to biotic agents) on the individual physiology, population distribution and dynamics (Régnière et al. 2012a; Gutierrez and Ponti 2013). The model was used to respond to the following assessment questions (AQ), which are highly relevant for estimating the risks linked to S. frugiperda in Europe (EFSA PLH Panel et al. 2018b). AQ 1—Is the model able to predict the pattern of population dynamics and the limits of establishment in the area of current distribution? (current distribution and dynamics); AQ 2—Can the species establish in Europe? If yes, what is the area of potential establishment of the species? (establishment in Europe); AQ 3—What is the population dynamics of the species in the areas of potential distribution in Europe? (population dynamics in Europe); AQ 4—Can the species originate transient populations in Europe? If yes, can population abundance in transient populations represent a risk for cultivated plants? (dynamics of transient populations).
Materials and methods
The model
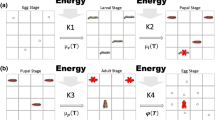
In this work, we developed a physiologically-based model using a system of Kolmogorov partial differential equations to simulate the stage-specific population dynamics of S. frugiperda considering the two dimensions of time \(t\) and physiological age \(x\) (Buffoni and Pasquali 2007; Rafikov et al. 2008; Solari and Natiello 2014; Lanzarone et al. 2017) (full mathematical details of the model are present in Section S1 of supplementary material 1). We assumed that a population of S. frugiperda is composed by four stages \(i\), namely egg (\(i = 1\)), larva (\(i = 2\)), pupa (\(i = 3\)), and adult (\(i = 4\)). Physiological age in the \(i\)-th stage, \(x^{i} \in \left[ {0,1} \right]\) represents the level of development of an individual in the stage \(i\) (Buffoni and Pasquali 2007). With \(x^{i} = 0\), we represent an individual at the beginning of \(i\)-th stage, while with \(x^{i} = 1\) we represent an individual at the end of the \(i\)-th stage. The term \(\phi^{i} \left( {t, \;x} \right)\) represents the number of individuals in stage \(i\) at time \(t\) with physiological age \(\left[ {x,\;x + {\text{d}}x} \right]\). The overall number of individuals in stage \(i\) at time \(t\) is calculated as \(N^{i} \left( t \right) = \mathop \smallint \limits_{0}^{1} \phi^{i} \left( {t, x} \right){\text{d}}x\). The population abundance in the stage \(i,\;N^{i} \left( t \right)\), is defined by the number of individuals in a spatial unit as defined in ‘Definition of the spatial unit’ section. In the present work, we considered the predicted adult population abundance (reported as the average number of moths per trap per week) as a descriptor of the potential impacts of S. frugiperda. The simulations were performed using MATLAB version R2018a (MATLAB, R2018a, The MathWorks, Inc., MA, USA). We assumed the population dynamics of S. frugiperda was dependent on the species’ life-history strategies. These were described at the individual level by stage-specific development, mortality, and fecundity rate functions. Since temperature is considered one of the main variables influencing the physiology of poikilotherms (Gutierrez 1996; Régnière et al. 2012b; Gilioli et al. 2021a), the effects of the time-dependent temperature profile \(T\left( t \right)\) affecting the species’ life-history strategies were considered in the model (Barfield et al. 1978; Silva et al. 2017; Du Plessis et al. 2020).
Development rate function
We defined \(\nu^{i} \left( {T\left( t \right)} \right)\) as the temperature-dependent development rate function of individuals in stage \(i\) as a function of temperature \(T\left( t \right)\). For the stages \(i = 1,2,3\) we used the development rate functions that are defined in Gilioli et al. (2021b). For the stage \(i = 4\), respect to Gilioli et al. (2021b), we increased the life-span of the adults by reducing the development rate function \(\nu^{4} \left( {T\left( t \right)} \right)\) by a fixed factor of 2.5 to obtain more realistic adult survival curves (He et al. 2021a; Zhang et al. 2021). The methodology used for estimating parameters of the development rate function \(\nu^{i} \left( {T\left( t \right)} \right)\) is presented in Section S1.1 of supplementary material 1.
Mortality rate function
As in Gilioli et al. (2021b), we assumed the mortality rate function \(m^{i} \left( t \right)\) for the stages \(i = 1,\; 3, \;4\) depending on temperature according to the following law
with \(\mu^{i} \left( {T\left( t \right)} \right)\) being the temperature-dependent instantaneous mortality acting on individuals within each stage at time \(t\) (see Section S1.2 of supplementary material 1). The mortality of larvae is affected by multiple factors, such as weather conditions (Varella et al. 2015), the attack of biotic agents (e.g. predators, parasites and pathogens) (Escribano et al. 2000; Zanuncio et al. 2008), and density-dependent factors (e.g. cannibalistic behaviour) (Chapman 1999; Chapman et al. 2000; Andow et al. 2015; He et al. 2021b). To account for these factors, in the present work, the mortality rate function for the larval stage \(m^{2} \left( t \right)\) is expressed as follows
with \(\left( {1 + \alpha \left( {\frac{{N^{2} \left( t \right)}}{\gamma }} \right)^{2} } \right)\) representing a density-dependent component simulating the intraspecific competition (e.g. cannibalistic behaviour), \(\alpha > 0\) representing a multiplicative term, and \(\beta > 0\) representing a biotic component simulating the role of predators, parasites and pathogens.
The parameter \(\gamma > 0\) represents the larval carrying capacity based on resources availability. The parameter \(\gamma\) was set to 3000 which corresponds to the larval abundance at the carrying capacity in the spatial unit considered in the present study (see ‘Definition of the spatial unit’ section for details). The methodology used for estimating the temperature-dependent component of the mortality rate function \(\mu^{i} \left( {T\left( t \right)} \right)\) is presented in Section S1.2 of supplementary material 1. Parameters \(\alpha\) and \(\beta\) were estimated through the calibration procedure (‘Model calibration’ section).
Fecundity rate function
For the adult stage, we defined the fecundity rate function \(F^{1} \left( t \right)\) representing the production of eggs by adult females (Johnson 1987). As in Gilioli et al. (2021b), the fecundity rate function depends on female age and temperature. In the present work, we further introduced a density-dependent regulation term to account for the role of intraspecific competition in egg production due to limitations in the per-capita food supply (Leather 2018). The fecundity rate used in the present study is
with \(g\left( {T\left( t \right)} \right)\) describing the temperature-dependent component, \(\left( {1 - \frac{{N^{4} \left( t \right)}}{{S + N^{4} \left( t \right)}}} \right)\) describing the density-dependent component, \(\phi^{4} \left( {t,x} \right)\) being the number of adult individuals at time \(t\) and physiological age \(x\), and \(h\left( x \right)\) describing the physiological age-dependent component. The terms \(g\left( {T\left( t \right)} \right)\) and \(h\left( x \right)\) were taken from Gilioli et al. (2021b) (see Section S1.3 of supplementary material 1 for details). The term \(S\) is a half-saturation term in the density-dependent regulation of female fecundity. Based on the assumption that the adult abundance at the carrying capacity in the spatial unit is \(N_{K}^{4} = 320\) adult individuals per week (see ‘Definition of the spatial unit’ section for details), we set \(S = 0.5 N_{K}^{4} = 160\). With this set up, the density-dependent term \(\left( {1 - \frac{{N^{4} \left( t \right)}}{{S + N^{4} \left( t \right)}}} \right)\) is almost 1.00 (negligible density-dependent effects) for adult population abundances lower than 10 individuals, and almost 0.35 (relevant density-dependent effects) for adult population abundances approaching 320 individuals per trap per week.
Model calibration
The calibration procedure consisted in estimating the parameter \(\alpha_{j}\) and \(\beta_{j}\) that will be used for the definition of the parameters \(\alpha\) of the density-dependent mortality term and the biotic mortality term \(\beta\) included in the mortality rate function of the larval stage \(m^{2} \left( t \right)\). Parameters \(\alpha_{j}\) and \(\beta_{j}\) were estimated by minimising the mean squared distance between the simulated and the observed adult abundance for each of the 21 observation datasets \(j\) representing the calibration dataset (see ‘Data on pest population dynamics’ section). The minimisation was performed for each of the 21 observation datasets \(j\) through solving the following function
The term \(A_{j} \left( {t_{i} } \right)\) represents the observed adult abundance in the dataset \(j\) at the time \(t_{i}\) corresponding to the time at which adult abundance was sampled. The term \(R_{j}\) represents the number of sampled data available for each dataset \(j\). With \(N_{j}^{4} \left( {t_{i} ;\alpha_{j} ,\beta_{j} } \right)\), we define the adult abundance in the dataset \(j\) at time \(t_{i}\), obtained by solving the Kolmogorov equations with the parameters \(\alpha = \alpha_{j}\) and \(\beta = \beta_{j}\) keeping fixed the other parameters. The optimal parameters \(\hat{a}_{j}\) and \(\hat{\beta }_{j}\) were the minimisers of the \(Q_{j}\), i.e. they allow for the minimum difference between simulated and observed adult population abundance
For the minimisation procedure, we used the MATLAB function fmincon with step tolerance equal to 10–5 for the stopping test.
Simulation design
The population dynamics model of S. frugiperda was used to explore the four assessment questions reported in ‘Introduction’ section. To account for the uncertainty linked to the estimates of parameters \(\alpha\) and \(\beta\), the model was implemented considering three assessment scenarios (see ‘Generation of assessment scenarios’ section).
Assessment question 1— Current distribution and dynamics
The capacity of the model to predict the local population dynamics of S. frugiperda was tested by comparing simulated and observed adult population abundance using data obtained in three locations selected along a latitudinal gradient in the area of current distribution in North America (see ‘Data on pest population dynamics’ section). From south to north, we considered a highly suitable location (Miami Dade County, Florida), a location at the edge of the area of establishment (Alachua County, Florida), and a location that is currently known to be reached only by migrating populations (Tift County, Georgia) (Westbrook et al. 2016; Garcia et al. 2018). The population dynamics were simulated using the temperature profile of the current climate in the tested locations as input data (see ‘Temperature data’ section). Initial conditions were set to 5 pupae uniformly distributed in their physiological age (from 0 to 1) on the 1st of January. The model was implemented for four consecutive years, repeating the same yearly temperature profile, to obtain stable population dynamic patterns and model outputs that were independent of the initial conditions. We assumed that no migration of individuals was possible from and to each location in which the model was implemented. The assessing variables considered were the yearly average number of moths per trap per week, the number of generations per year, and the maximum adult population abundance reached over the last year of simulation.
Assessment question 2— Establishment in Europe
For assessing the potential distribution and abundance of S. frugiperda in Europe, we implemented the model in a spatial grid of 0.1° × 0.1° representing the European territory (see ‘Temperature data’ section). In each node of the grid, the population dynamics was assessed using the same initial conditions defined in AQ 1 and the temperature profile of that specific node (Gilioli et al. 2014, 2021c; Pasquali et al. 2020). The species was considered established in a node if, at the first time-step of January 1st of the last year of simulation, the adult abundance was higher than an adult abundance threshold \(\left( {A_{0} = 0.01} \right)\). The threshold \(A_{0}\) was set by considering the average of the minimum population abundance reached by the species at the northernmost edge of the area of establishment in a set of locations in North America, including the location of Alachua County (Florida) tested in AQ 1. Species’ potential distribution was estimated over the last year of simulation. The area of potential establishment of S. frugiperda in Europe was given by the set of grid nodes where the species was considered established.
Assessment question 3— Population dynamics in Europe
The local population dynamics of S. frugiperda in Europe was assessed by implementing the model in 3 locations using the initial conditions explained in AQ 1. Locations were chosen based on the simulated S. frugiperda potential dynamics in Europe obtained by answering AQ 2. Based on the model’s result, a highly suitable location was selected in Cyprus, and two less suitable locations were selected, in Southern France and on the Atlantic coast of Portugal. We considered the same assessing variables presented in AQ 1.
Assessment question 4— Dynamics of transient populations
Transient populations are analysed in a hypothetical scenario in which migrating adults arrive in a location characterised by temporary suitable conditions (e.g. warm temperature conditions during spring or summer), but where the species is not able to survive during fall or winter. To assess the dynamics of transient populations, we simulated the introduction of an inoculum characterised by five adult individuals uniformly distributed in their physiological age (from 0 to 1) in four maize production areas in Europe, outside the predicted area of establishment: Rădoiești (Romania, 44th parallel north), Ghedi (Italy, 45th parallel north), Ouarville (France, 48th parallel north), and Engelsberg (Germany, 48th parallel north). The dynamics of transient populations was assessed considering three different Days of the Year (DOY) for the introduction of the inoculum: April 1 (90th DOY), June 1 (150th DOY), and August 1 (210th DOY). The model was implemented from the date of introduction of the inoculum to the end of the year, using as temperature profile the current climate in the tested location (see ‘Temperature data’ section). The assessing variables considered were the average number of moths per trap per week, the number of generations, and the maximum adult population abundance over the simulation period. We assumed that the inoculum was not able to originate a transient population if the predicted adult population abundance reached values below or equal to the adult abundance threshold \(A_{0}\) during the simulation period.
Generation of assessment scenarios
Considering the range of distribution of the parameters \(\alpha_{j}\) and \(\beta_{j}\) estimated through the calibration procedure (see ‘Model calibration’ section), we calculated the 10th, the 50th, and the 90th quantiles of the distributions for the definition of parameters \(\alpha\) and \(\beta\). To account for variability in the population dynamics, we generated 9 different assessment scenarios, combining the quantiles of \(\alpha\) and \(\beta\). In the present study, we consider the worst-case assessment scenario where the species has lower mortality \(\left( {\alpha = 10{\text{th; }}\beta = 10th} \right)\), The median-case assessment scenario, obtained considering the medians of parameters distribution \(\left( {\alpha = 50th;\;\beta = 50th} \right)\), and the best-case assessment scenario, where S. frugiperda mortality is high \(\left( {\alpha = 90th;\;\beta = 90th} \right)\). The values of parameters related to the three investigated scenarios are reported in Table 1. The population dynamics of S. frugiperda in the current area of establishment in North America (AQ 1) and the dynamics of transient populations in Europe (AQ 4) were predicted considering the median-case assessment scenario. The best-case, the median-case, and the worst-case assessment scenarios were considered for predicting the potential distribution of S. frugiperda in Europe (AQ 2) and the population dynamics of the pest within the predicted area of establishment (AQ 3).
Data
Data on pest population dynamics
Data on pest population dynamics were used for estimating parameters in the function describing the larval mortality (see ‘Model calibration’ section) and to test the model’s capacity to predict the population dynamics patterns and the establishment of S. frugiperda in North America (AQ 1). Population dynamics data used for calibration purposes (hereinafter, calibration dataset) refer to 21 time-series adult trap catches data collected in the area of establishment in Central and North America from 1982 to 2019, selected for their quality in terms of completeness of the time-series and realism of population trends (see supplementary material 2) (Silvain and Ti-A-Hing 1985; Pair et al. 1986; Nagoshi and Meagher 2004; Meagher and Nagoshi 2004; Rojas et al. 2004; Salas-Araiza et al. 2018; Salazar-Blanco et al. 2020). The calibration dataset covers latitudes between 4.85 and 28.76 parallel north and it includes, 1 time-series data collected in French Guyana (Matoury), 1 in Costa Rica (Guanacaste), 3 in Mexico (Manzano and Irapuato), and 16 in the United States (from Southern to Northern Florida). Additional population dynamics data were used for answering the AQ 1 and refer to time-series adult trap catches collected in three locations: Miami Dade County (Florida, 25th parallel north), Alachua County (Florida, 29th parallel north), and Tift County (Georgia, 31st parallel north) (Pair et al. 1986; Meagher and Nagoshi 2004; Garcia et al. 2019).
Definition of the spatial unit
The simulated adult abundance variable used in our model \(N^{4} \left( t \right)\) refers to the number of adult individuals caught in a trap per week. To consistently allow the comparison between observed and simulated adult population abundance, the temporal unit of the population dynamics data used in the present study was referring to weekly adult trap catches. Since a pheromone-baited trap can effectively catch insects within a range of two hectares (Tingle and Mitchell 1979), the spatial unit for the definition of the adult population abundance was considered two hectares in the present study. Our model required the estimation of the larval carrying capacity \({ }\gamma\). Considering the whole calibration dataset, we first calculated the average maximum observed adult abundance (284 individuals per trap per week). Based on this result we assumed a conservative value representing the carrying capacity of the adults in the spatial unit \(N_{K}^{4} = 320\). The relation between the seasonal fluctuations of adults (captured using pheromone-baited traps) and larvae (captured using sweep nets) of S. frugiperda was investigated for three consecutive years (1981–1983) by Silvain and Ti-A-Hing (1985). From their work, we extracted 10 datasets and calculated the average amount of larvae produced by a single adult (i.e. the ratio between larval and adult abundance at the peaks of the population) \(P = 9.34\). Based on this result, we calculated the carrying capacity of larvae \(\gamma = N_{K}^{4} P = 2989\) which was rounded to \(\gamma = 3000\) in the present study.
Data on species physiology
The development \(\nu^{i} \left( {T\left( t \right)} \right)\), mortality \(m^{i} \left( t \right)\) and fecundity \(F^{1} \left( t \right)\) rate functions were estimated considering data available in the literature on stage-specific responses of S. frugiperda exposed to different constant temperature conditions. Data referring to the average stage-specific duration in days were used for estimating the development rate function \(\nu^{i} \left( {T\left( t \right)} \right)\) (Barfield et al. 1978; Simmons 1993; Oeh et al. 2001; Busato et al. 2005; Milano et al. 2008; Barros et al. 2010; Ríos-Díez and Saldamando-Benjumea 2011; Garcia et al. 2018). Data referring to the stage-specific percentage survival were used for estimating the temperature-dependent component \(\mu^{i} \left( {T\left( t \right)} \right)\) of the mortality rate function \(m^{i} \left( t \right)\) (Barfield et al. 1978; Pashley et al. 1995; Murúa and Virla 2004; Busato et al. 2005; Milano et al. 2008; Barros et al. 2010; Garcia et al. 2018). Data referring to the temperature-dependent average total fecundity, average daily fecundity, and average duration in days of the oviposition period were used for estimating the temperature- \(g\left( {T\left( t \right)} \right)\) and the physiological age-dependent \(h\left( x \right)\) components of the fecundity rate function \(F^{1} \left( t \right)\) (Barfield et al. 1978; Pashley et al. 1995; Oeh et al. 2001; Milano et al. 2008; Barros et al. 2010; Garcia et al. 2018).
Temperature data
Yearly temperature data used as inputs during model calibration refer to the 5th generation of European ReAnalysis (ERA5-Land), reporting hourly air temperature data at a 0.1° × 0.1° spatial resolution (Muñoz Sabater 2019). Bilinear interpolation was used to obtain temperature data for each location of the calibration dataset. The current climatic scenario used to respond to the assessment questions was extracted from the Coordinated Regional Downscaling Experiment (CORDEX) (Jacob et al. 2014) and refers to the Coupled Model Intercomparison Project Phase 5 (CMIP5). The scenario is based on Representative Concentration Pathways (RCPs) which consider the greenhouse gases emissions up to the year 2100 (van Vuuren et al. 2011). The climatic scenario provides tri-hourly temperature data on 0.11° × 0.11° spatial resolution for the European domain over a period ranging between 2016 and 2025. Temperature data were regridded through bilinear interpolation to a regular 0.1° × 0.1° grid using Climate Data Operators command lines (Schulzweida 2019). We then averaged tri-hourly data over the whole decade (2016–2025) of the scenario to obtain an annual average temperature profile, which was assumed as the current climate (see Section S2 of supplementary material 1).
Results
Below are presented the answers to the four assessment questions, based on the results of the model.
AQ 1— Predicted population dynamics and limits of establishment of Spodoptera frugiperda in areas of current distribution
The graphical results of the model implemented along a south–north latitudinal gradient under the median-case assessment scenario are presented in Fig. 1. In the area of Miami Dade (Florida), the model predicted seven peaks (i.e. generations) per year; the predicted yearly average number of moths per trap per week was around 64 individuals and the maximum adult population abundance was around 165 individuals reached on the 6th generation. In the area of Alachua (Florida), the model predicted two generations per year; the yearly average adult abundance was around 17 individuals, and the maximum adult population abundance was around 98 individuals reached on the 2nd generation. Adult population abundance reached values lower than the adult population threshold \(A_{0}\) over the simulation period in the area of Tift (Georgia). Thus, the potential establishment of the pest was considered not possible in the above-mentioned area.
AQ 2— Risk of establishment and potential distribution of Spodoptera frugiperda in Europe
Figures 2 and 3 show the risks of establishment of S. frugiperda in Europe under the three assessment scenarios. In the median-case scenario, risk of establishment was predicted in the southern coastal areas of the Mediterranean basin (Cyprus, Syria, Lebanon, Southern Turkey, Southern Italy, Southern, and Western Spain). A lower risk of establishment was expected in the Atlantic coasts of Portugal, and sporadic locations on the west coast of Sardinia. In the median-case assessment scenario, the measured area of potential establishment was 0.26% of the whole area under assessment. The area of establishment decreased by 89% (0.03% of the total assessed area) in the best-case assessment scenario and increased by 116% (0.57% of the total assessed area) in the worst-case assessment scenario. The northernmost latitudinal limit marking the presence of S. frugiperda populations was the 38th parallel north (Eastern Spain), the 43rd parallel north (Southern France), and the 44th parallel north (Northern Italy) in the best-case, median-case, and worst-case assessment scenarios, respectively.
AQ 3— Predicted population dynamics of Spodoptera frugiperda in areas of potential distribution in Europe
Estimated population abundance within the area of potential establishment in Europe was highly variable depending on the assessment scenarios. The predicted yearly average number of moths per trap per week (± standard deviation) in the spatial unit was 5 (± 4) in the best-case, 17 (± 5) in the median-case, and 139 (± 22) in the worst-case assessment scenario. More details on the yearly population dynamic patterns of S. frugiperda are provided by the results of the local implementation of the model in areas with different suitability for the species in Europe (Fig. 4). The results of the model implemented in a highly suitable area (Cyprus) showed low population abundances at the beginning of the year due to low temperatures. Approaching the spring season, a rise in the adult population abundance was predicted according to temperature increase. Four adult population peaks (i.e. generations) were predicted around the 186th, 225th, 264th, and 314th DOY with the maximum adult population abundance reached on the third generation. Predicted adult population abundances during the peaks ranged between 90 and 130 individuals. After the fourth generation, a decline in the abundance of adults was observed, due to temperature drops in fall. The model implemented in the northernmost edge of the predicted establishment area in Europe (La Seyne-sur-Mer, Southern France) resulted in a single generation at around the 250th DOY. Adult abundance reached 90 individuals and then a sharp drop in population abundance was observed due to cold temperatures in fall. The model implemented in the Atlantic coast of Portugal (Alcochete) showed a single generation at around the 297 DOY with low adult population abundance during the peak (18 individuals).
AQ 4— Risks linked to transient populations of Spodoptera frugiperda in Europe
Simulations of the inoculum in different periods of the year (considering the median-case assessment scenario) clearly showed that the species might be able to establish transient populations outside the predicted establishment area in Europe (Fig. 5). The results of the model implemented in Rădoiești (Southern Romania, 44th parallel north) and Ghedi (Northern Italy, 45th parallel north) showed risks linked to transient populations in all three introduction periods. A single generation was predicted for introductions at the 90th DOY, and at the 210th DOY and two generations were expected for introductions at the 150th DOY when weather conditions can be particularly suitable for the species. The predicted average number of moths per trap per week ranged between 19 and 20 adults with peaks at around 70 individuals (introduction at the 90th DOY) to 43–52 individuals (introductions at the 150th and 210th DOY). The model was implemented in areas further north in Europe (48th parallel north) in Ouarville (Northern France) and Engelsberg (Southern Germany). Introductions at the 90th DOY did not represent a risk of transient populations (\(N^{4} < A_{0}\) during the simulation period) due to the unsuitable environmental conditions affecting species’ survival. Introductions occurring during warmer periods in late spring (150th DOY) and summer (210th DOY) allowed the species to originate transient populations. However, only low yearly average adult population abundances (1–2 individuals) were predicted, thus representing a low risk linked to transient populations.
Population dynamics of adults of Spodoptera frugiperda simulating an inoculum of 5 adults at the 90th, (green line), 150th (blue line), and 210th (red line) day of the year in (upper left corner) Rădoiești (Southern Romania), (upper right corner) Ghedi (Southern Italy), (lower left corner) Ouarville (Northern France) and (lower right corner) Engelsberg (Germany)
Discussion
The model presented was able to satisfactorily predict the population dynamics, the variability in the number of generations, and the limits in the area of establishment of S. frugiperda along a latitudinal gradient within the area of current distribution in North America. The model implemented under the median-case assessment scenario predicted up to seven generations per year in an area where the species is well established (Florida, Miami Dade County) and only two generations per year in a location situated at the northernmost edge of the establishment for the species (Florida, Alachua County). These results are in agreement with the available observations reporting a high population abundance and population dynamics characterised by continuous generations throughout the year in tropical areas of Central America characterised by warmer temperature conditions (Sparks 1979; Busato et al. 2005), and around six generations in warm areas of North America (Luginbill 1928). According to the results of the model, the number of generations progressively decreases moving towards the northern areas of distribution of the species (Johnson 1987; Ramirez-Cabral et al. 2017; Schlemmer 2018). Correctly, the model predicted no establishment in areas (Tift County, Georgia) considered reached only by migratory populations. These results highlight the prominent role of climate in influencing the distribution and the dynamics of S. frugiperda (Capinera 2002; Garcia et al. 2018, 2019).
The predicted distribution and dynamics of S. frugiperda in Europe clearly highlighted risks of establishment of the species, especially in the coastal areas of the Mediterranean basin due to more favourable climatic conditions. In particular, higher average adult population abundances were predicted in the coastal areas of Southern Spain, Southern Italy, Greece, Cyprus, Southern Turkey, and Lebanon. Our results are in partial disagreement with the work of Du Plessis et al. (2018), which reported a low risk of establishment, but mainly risk linked to transient populations, associated with the pest in Europe. In agreement with our results, suitable areas for the establishment of S. frugiperda in the Mediterranean coasts of Europe have been reported in EFSA PLH Panel et al. (2018a), Early et al. (2018), Liu et al. (2020), Baloch et al. (2020), Zacarias (2020), and Tepa-Yotto et al. (2021). The predicted northernmost limit that might be reached by S. frugiperda was between the 38th and the 44th parallel north based on the different scenarios under assessment. These results are in partial disagreement with Liu et al. (2020), Baloch et al. (2020), Fan et al. (2020), and Tepa-Yotto et al. (2021). These authors reported areas potentially suitable for the establishment of the species further north, reaching the United Kingdom and Southern Sweden (although with low habitat suitability indices). Current knowledge of the biology of S. frugiperda seems to justify our predictions, especially in the light of the prominent role of climate in shaping the area of distribution of the species (Early et al. 2018). In particular, it is reported that the species does not enter diapause and suffers from cold weather conditions (Capinera 2002; Nagoshi et al. 2012). This might prevent the establishment of S. frugiperda in cold areas (EFSA PLH Panel et al. 2018a).
The population dynamics pattern of S. frugiperda predicted by the model in a highly suitable location in Europe showed that a rise in the adult population abundance (up to 90–130 adult individuals per trap per week) may occur during the early summer period, with up to four generations per year. A single generation per year and lower population abundances (around 18–90 adult individuals per trap per week) were predicted in less suitable locations in Europe. This result is in agreement with EFSA PLH Panel et al. (2018a) which reported up to four generations per year in the most suitable areas in Southern Europe. The population pressure expected in a suitable European location might represent a risk for local crop production.
Given the high migratory ability of the species, transient populations might represent a threat in areas outside the area of potential establishment of the species. The results of the model showed that in Europe, high risk due to transient populations can be expected in areas up to the 45th parallel north, with adult population abundances (20–70 adult individuals per trap per week) that might cause impacts on local crop production. Lower risks due to transient populations in Europe can be expected in areas up to the 48th parallel north, where unsuitable climatic conditions hinder the survival of the inoculum.
The physiologically-based modelling approach used in the present study requires the definition of biologically meaningful parameters describing the life-history of S. frugiperda. Parameters related to the temperature-dependent components influencing development, mortality, and fecundity were easily estimated using the large amount of data available in the literature. Conversely, the lack of data linked to density-dependent effects on adult fecundity and larval mortality forced us to make reasonable assumptions in the mathematical description of these components. Parameters representing density-dependent and biotic regulation affecting larval mortality have been calibrated using time-series population dynamics data. Parameters might be further fine-tuned if more data become available. In our model, temperature is the only abiotic variable influencing biological processes. If relevant, the model might be easily extended to include the influence of other environmental variables, such as relative humidity. The model does not consider the influence of different host plant species on the life-history (Chen et al. 2022), the dynamics, and the distribution of S. frugiperda (Baloch et al. 2020). Another source of uncertainty not considered in the model is represented by variability in the physiological responses associated with different strains of the species that might reach Europe (Sarr et al. 2021). The scenario-based approach we implemented seeks to cover part of the issues linked to parameters’ estimates and model’s limitations.
Conclusion
In this work, we present the results of a physiologically-based model applied to S. frugiperda to (i) predict the species’ population dynamics and abundance, (ii) assess the risk of establishment of the species in Europe, and (iii) predict the risk linked to transient populations in Europe. To the best of our knowledge, this is the first physiologically-based model simulating the life-history of S. frugiperda used for investigating the potential dynamics and distribution of the species in Europe. The physiologically-based modelling approach allowed us to simulate the influence of biotic (density-dependent effects and mortality due to biotic agents) and abiotic (e.g. temperature) variables on the life-history strategies of a pest (Soberon and Nakamura 2009; Gutierrez and Ponti 2013). This approach provides realistic predictions that are independent of data on the current distribution of the species that might be incomplete and/or biased (Wiens et al. 2009).
The model presented can provide fundamental elements for supporting the management of the pest considering different spatio-temporal scales and management contexts (Sperandio 2021). In case S. frugiperda is still absent from a territory, preventive measures should be taken, namely pest risk analysis (PRA), update of phytosanitary regulations (including the potential for response measures), inspection and diagnostics, and surveillance (EU 2018; FAO 2021). The quantitative outputs of the model (e.g. average population abundance, population dynamics, and potential distribution of the pest) provide fundamental information for the analysis of the risks posed by S. frugiperda in Europe. Risk maps generated by the model can be used to guide the implementation of detection surveys in the identification of high-risk areas that might be particularly suitable for the establishment of the pest. Similarly, the definition of the frequency and the intensity of the inspection measures can be guided by coupling risk maps on the potential establishment of the pest and information on trade routes and movement of people (EFSA PLH Panel et al. 2018b; FAO 2021). The model also provides relevant information on the potential impacts caused by transient populations that might represent a risk for local crop production. In case the species becomes established in mainland Europe, predictions on the population phenology and dynamics of S. frugiperda can be used for the timely implementation of control actions aimed at reducing pest population pressure and thus reducing the impacts on local crops (Rossi et al. 2019).
Authors’ contribution
GG and GS conceptualised the work. GG, GS, AS, and PG performed simulations. GG, GS, AS, and PG, interpreted outputs. GG, GS, and MC acquired and interpreted data. All authors drafted the manuscript.
Data availability
Raw data used in the present study are available upon request.
References
Andow DA, Farias JR, Horikoshi RJ et al (2015) Dynamics of cannibalism in equal-aged cohorts of Spodoptera frugiperda. Ecol Entomol 40:229–236. https://doi.org/10.1111/een.12178
Baloch MN, Fan J, Haseeb M, Zhang R (2020) Mapping potential distribution of Spodoptera frugiperda (Lepidoptera: Noctuidae) in Central Asia. Insects 11:172. https://doi.org/10.3390/insects11030172
Barfield CS, Mitchell ER, Poeb SL (1978) A temperature-dependent model for fall armyworm development. Ann Entomol Soc Am 71:70–74. https://doi.org/10.1093/aesa/71.1.70
Barros EM, Torres JB, Bueno AF (2010) Oviposição, desenvolvimento e reprodução de Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae) em diferentes hospedeiros de importância econômica. Neotrop Entomol 39:996–1001. https://doi.org/10.1590/S1519-566X2010000600023
Baudron F, Zaman-Allah MA, Chaipa I et al (2019) Understanding the factors influencing fall armyworm (Spodoptera frugiperda J.E. Smith) damage in African smallholder maize fields and quantifying its impact on yield. A case study in Eastern Zimbabwe. Cro Prot 120:141–150. https://doi.org/10.1016/j.cropro.2019.01.028
Buffoni G, Pasquali S (2007) Structured population dynamics: continuous size and discontinuous stage structures. J Math Biol 54:555–595. https://doi.org/10.1007/s00285-006-0058-2
Busato GR, Grützmacher AD, Garcia MS et al (2005) Thermal requirements and estimate of the number of generations of biotypes “corn” and “rice” of Spodoptera frugiperda. Pesqui Agropecu Bras 40:329–335. https://doi.org/10.1590/S0100-204X2005000400003
Capinera JL (2002) Fall armyworm, Spodoptera frugiperda (JE Smith) (Insecta: Lepidoptera: Noctuidae): EENY098/IN255, rev. 7/2000. EDIS. https://doi.org/10.32473/edis-in255-2000
Chapman JW (1999) Fitness consequences of cannibalism in the fall armyworm, Spodoptera frugiperda. Behav Ecol 10:298–303. https://doi.org/10.1093/beheco/10.3.298
Chapman JW, Williams T, Martínez AM et al (2000) Does cannibalism in Spodoptera frugiperda (Lepidoptera: Noctuidae) reduce the risk of predation? Behav Ecol Sociobiol 48:321–327. https://doi.org/10.1007/s002650000237
Chen Y-C, Chen D-F, Yang M-F, Liu J-F (2022) The effect of temperatures and hosts on the life cycle of Spodoptera frugiperda (Lepidoptera: Noctuidae). Insects 13:211. https://doi.org/10.3390/insects13020211
Cock MJW, Beseh PK, Buddie AG et al (2017) Molecular methods to detect Spodoptera frugiperda in Ghana, and implications for monitoring the spread of invasive species in developing countries. Sci Rep 7:4103. https://doi.org/10.1038/s41598-017-04238-y
da Silva DM, de Bueno AF, Andrade K et al (2017) Biology and nutrition of Spodoptera frugiperda (Lepidoptera: Noctuidae) fed on different food sources. Sci agric Piracicaba Braz 74:18–31. https://doi.org/10.1590/1678-992x-2015-0160
Day R, Abrahams P, Bateman M et al (2017) Fall armyworm: impacts and implications for Africa. Outlook Pest Manag 28:196–201. https://doi.org/10.1564/v28_oct_02
de Freitas Bueno RCO, de Freitas BA, Moscardi F et al (2011) Lepidopteran larva consumption of soybean foliage: basis for developing multiple-species economic thresholds for pest management decisions. Pest Manag Sci 67:170–174. https://doi.org/10.1002/ps.2047
Devi S (2018) Fall armyworm threatens food security in southern Africa. Lancet 391:727. https://doi.org/10.1016/S0140-6736(18)30431-8
Du Plessis H, Schlemmer M-L, Van den Berg J (2020) The effect of temperature on the development of Spodoptera frugiperda (Lepidoptera: Noctuidae). Insects 11:228. https://doi.org/10.3390/insects11040228
Du Plessis H, Van den Berg J, Ota N, Kriticos DJ (2018) Spodoptera frugiperda. Fall Armyworm, CLIMEX modelling CSIRO-InSTePP Pest Geography. https://pra.eppo.int/pra/1337e7c5-1744-4a2b-a782-b344ad06d4e6. Accessed 14 Mar 2021
Early R, González-Moreno P, Murphy ST, Day R (2018) Forecasting the global extent of invasion of the cereal pest Spodoptera frugiperda, the fall armyworm. NeoBiota 40:25–50. https://doi.org/10.3897/neobiota.40.28165
EFSA, Kinkar M, Delbianco A, Vos S (2020) Pest survey card on Spodoptera frugiperda. EFSA J 17:7. https://doi.org/10.2903/sp.efsa.2020.EN-1895
EFSA PLH Panel, Jeger M, Bragard C et al (2017) Pest categorisation of Spodoptera frugiperda. EFSA J 15:7. https://doi.org/10.2903/j.efsa.2017.4927
EFSA PLH Panel, Jeger M, Bragard C et al (2018a) Pest risk assessment of Spodoptera frugiperda for the European Union. EFSA J 16:8. https://doi.org/10.2903/j.efsa.2018.5351
EFSA PLH Panel, Jeger M, Bragard C et al (2018b) Guidance on quantitative pest risk assessment. EFSA J 16:e05350. https://doi.org/10.2903/j.efsa.2018.5350
EPPO (2019a) EPPO reporting service 2019/136. First report of Spodoptera frugiperda in Egypt. https://gd.eppo.int/reporting/article-6566. Accessed 15 Mar 2021
EPPO (2019b) EPPO reporting service 2019/053. Spodoptera frugiperda continues to spread in Asia. https://gd.eppo.int/reporting/article-6483. Accessed 17 Mar 2021
EPPO (2019c) EPPO reporting service 2019/006. First report of Spodoptera frugiperda in Thailand. https://gd.eppo.int/reporting/article-6436. Accessed 17 Mar 2021
EPPO (2019d) EPPO reporting service 2019d/029. First report of Spodoptera frugiperda in China. https://gd.eppo.int/reporting/article-6459. Accessed 18 Mar 2021
EPPO (2019e) EPPO reporting service 2019/138. First report of Spodoptera frugiperda in Japan. https://gd.eppo.int/reporting/article-6568. Accessed 12 Dec 2021
EPPO (2020a) EPPO reporting service 2020/143. First report of Spodoptera frugiperda in Mauritania. https://gd.eppo.int/reporting/article-6821. Accessed 13 Dec 2021
EPPO (2020b) EPPO reporting service 2020/213. First report of Spodoptera frugiperda in Jordan. https://gd.eppo.int/reporting/article-6891. Accessed 13 Apr 2021
EPPO (2020c) EPPO reporting service 2020/161. First report of Spodoptera frugiperda in Israel. https://gd.eppo.int/reporting/article-6839. Accessed 27 Apr 2021
EPPO (2020d) EPPO Reporting service 2020/092. First report of Spodoptera frugiperda in United Arab Emirates. https://gd.eppo.int/reporting/article-6770. Accessed 21 May 2021
EPPO (2020e) EPPO reporting service 2020/142. First report of Spodoptera frugiperda in Timor-Leste. https://gd.eppo.int/reporting/article-6820. Accessed 22 Dec 2021
EPPO (2020f) EPPO reporting service 2020/031. First report of Spodoptera frugiperda in Australia. https://gd.eppo.int/reporting/article-6709. Accessed 12 Mar 2021
EPPO (2018) EPPO reporting service 2018/154. First report of Spodoptera frugiperda in India. https://gd.eppo.int/reporting/article-6348. Accessed 12 Mar 2021
EPPO (2021) EPPO reporting service 2021/053. First report of Spodoptera frugiperda in the Canary Islands, Spain. https://gd.eppo.int/reporting/article-6992. Accessed 13 Dec 2021
Escribano A, Williams T, Goulson D et al (2000) Parasitoid–pathogen–pest interactions of Chelonus insularis, Campoletis sonorensis, and a nucleopolyhedrovirus in Spodoptera frugiperda larvae. Biol Control 19:265–273. https://doi.org/10.1006/bcon.2000.0865
EU (2018) Commission decision (EU) 2018/638 of 23 april 2018 establishing emergency measures to prevent the introduction into and the spread within the Union of the harmful organism Spodoptera frugiperda (Smith). OJ L 105:31–34. https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32018D0638. Accessed 21 Mar 2021
EU (2019) Commission delegated regulation (EU) 2019/1702 of 1 august 2019 supplementing regulation (EU) 2016/2031 of the European parliament and of the council by establishing the list of priority pests. OJ L 260:8–10. https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32019R1702. Accessed 21 Mar 2021
Fan J, Wu P, Tian T et al (2020) Potential distribution and niche differentiation of Spodoptera frugiperda in Africa. Insects 11:383. https://doi.org/10.3390/insects11060383
FAO (2020) The global action for fall armyworm control: action framework 2020–2022—working together to tame the global threat. FAO, Rome, Italy. https://doi.org/10.4060/ca9252en
FAO (2021) Prevention, preparedness and response guidelines for Spodoptera frugiperda. FAO on behalf of the Secretariat of the International Plant Protection Convention. https://doi.org/10.4060/cb5880en
Farias CA, Brewer MJ, Anderson DJ et al (2014) Native maize resistance to corn earworm, Helicoverpa zea, and fall armyworm, Spodoptera frugiperda, with notes on aflatoxin content. Southwest Entomol 39:411–426. https://doi.org/10.3958/059.039.0303
Garcia AG, Godoy WAC, Thomas JMG et al (2018) Delimiting strategic zones for the development of fall armyworm (Lepidoptera: Noctuidae) on corn in the state of Florida. J Econ Entomol 111:120–126. https://doi.org/10.1093/jee/tox329
Garcia AG, Ferreira CP, Godoy WA, Meagher RL (2019) A computational model to predict the population dynamics of Spodoptera frugiperda. J Pest Sci 92:429–441. https://doi.org/10.1007/s10340-018-1051-4
Gilioli G, Pasquali S, Parisi S, Winter S (2014) Modelling the potential distribution of Bemisia tabaci in Europe in light of the climate change scenario. Pest Manag Sci 70:1611–1623. https://doi.org/10.1002/ps.3734
Gilioli G, Sperandio G, Simonetto A et al (2021) Modelling diapause termination and phenology of the Japanese beetle Popillia japonica. J Pest Sci. https://doi.org/10.1007/s10340-021-01434-8
Gilioli G, Colli P, Colturato M et al (2021b) A nonlinear model for stage-structured population dynamics with nonlocal density-dependent regulation: an application to the fall armyworm moth. Math Biosci 335:108573. https://doi.org/10.1016/j.mbs.2021.108573
Gilioli G, Sperandio G, Colturato M et al (2021c) Non-linear physiological responses to climate change: the case of Ceratitis capitata distribution and abundance in Europe. Biol Invasions. https://doi.org/10.1007/s10530-021-02639-9
Goergen G, Kumar PL, Sankung SB et al (2016) First report of outbreaks of the fall armyworm Spodoptera frugiperda (J E Smith) (Lepidoptera, Noctuidae), a new alien invasive pest in West and Central Africa. PLoS ONE 11:e0165632. https://doi.org/10.1371/journal.pone.0165632
Gutierrez AP (1996) Applied population ecology: a supply-demand approach. John Wiley & Sons
Gutierrez AP, Ponti L (2013) Eradication of invasive species: why the biology matters. Environ Entomol 42:395–411. https://doi.org/10.1603/EN12018
Hardke JT, Lorenz GM, Leonard BR (2015) Fall armyworm (Lepidoptera: Noctuidae) ecology in southeastern cotton. J Integr Pest Manag 6:10–10. https://doi.org/10.1093/jipm/pmv009
Harrison FP (1984) The development of an economic injury level for low populations of fall armyworm (Lepidoptera: Noctuidae) in grain corn. Fla Entomol 67:335. https://doi.org/10.2307/3494710
He L, Zhao S, Ali A et al (2021a) Ambient humidity affects development, survival, and reproduction of the invasive fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae), in China. J Econ Entomol 114:1145–1158. https://doi.org/10.1093/jee/toab056
He H, Zhou A, He L et al (2021b) The frequency of cannibalism by Spodoptera frugiperda larvae determines their probability of surviving food deprivation. J Pest Sci. https://doi.org/10.1007/s10340-021-01371-6
Hruska AJ, Gould F (1997) Fall armyworm (Lepidoptera: Noctuidae) and Diatraea lineolata (Lepidoptera: Pyralidae): impact of larval population level and temporal occurrence on maize yield in Nicaragua. J Econ Entomol 90:611–622. https://doi.org/10.1093/jee/90.2.611
Huang Y, Dong Y, Huang W et al (2020) Overwintering distribution of fall armyworm (Spodoptera frugiperda) in Yunnan, China, and influencing environmental factors. Insects 11:805. https://doi.org/10.3390/insects11110805
Jacob D, Petersen J, Eggert B et al (2014) EURO-CORDEX: new high-resolution climate change projections for European impact research. Reg Environ Change 14:563–578. https://doi.org/10.1007/s10113-013-0499-2
Johnson SJ (1987) Migration and the life history strategy of the fall armyworm, Spodoptera frugiperda in the western hemisphere. Int J Trop Insect Sci 8:543–549. https://doi.org/10.1017/S1742758400022591
Koffi D, Kyerematen R, Eziah VY et al (2020) Assessment of impacts of fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae) on maize production in Ghana. J Integr Pest Manag 11:20. https://doi.org/10.1093/jipm/pmaa015
Kumela T, Simiyu J, Sisay B et al (2019) Farmers’ knowledge, perceptions, and management practices of the new invasive pest, fall armyworm (Spodoptera frugiperda) in Ethiopia and Kenya. Int J Pest Manag 65:1–9. https://doi.org/10.1080/09670874.2017.1423129
Lanzarone E, Pasquali S, Gilioli G, Marchesini E (2017) A Bayesian estimation approach for the mortality in a stage-structured demographic model. J Math Biol 75:759–779. https://doi.org/10.1007/s00285-017-1099-4
Leather SR (2018) Factors affecting fecundity, fertility, oviposition, and larviposition in insects. Insect reproduction. CRC Press, pp 143–174
Liu T, Wang J, Hu X, Feng J (2020) Land-use change drives present and future distributions of fall armyworm, Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae). Sci Total Environ 706:135872. https://doi.org/10.1016/j.scitotenv.2019.135872
Luginbill P (1928) The fall army worm. US Department of Agriculture
Meagher RL, Nagoshi RN (2004) Population dynamics and occurrence of Spodoptera frugiperda host strains in southern Florida. Ecol Entomol 29:614–620. https://doi.org/10.1111/j.0307-6946.2004.00629.x
Milano P, Berti Filho E, Parra JR, Consoli FL (2008) Temperature effects on the mating frequency of Anticarsia gemmatalis Hüebner and Spodoptera frugiperda (JE Smith)(Lepidoptera: Noctuidae). Neotrop Entomol 37:528–535. https://doi.org/10.1590/s1519-566x2008000500005
Montezano DG, Specht A, Sosa-Gómez DR et al (2018) Host plants of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas. Afr Entomol 26:286–300. https://doi.org/10.4001/003.026.0286
Muñoz Sabater J (2019) ERA5-Land hourly data from 1981 to present. Copernicus Climate change service (C3S) Climate data store (CDS). https://doi.org/10.24381/cds.e2161bac. Accessed 9 Mar 2021
Murúa G, Virla E (2004) Population parameters of Spodoptera frugiperda (Smith) (Lep.: Noctuidae) fed on corn and two predominant grasess in Tucuman (Argentina). Acta Zool Mex 20:199–210. http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S0065-17372004000100015. Accessed 12 Mar 2021
Nagoshi RN, Meagher RL (2004) Seasonal distribution of fall armyworm (Lepidoptera: Noctuidae) host strains in agricultural and turf grass habitats. Environ Entomol 33:881–889. https://doi.org/10.1603/0046-225X-33.4.881
Nagoshi RN, Adamczyk JJ, Meagher RL et al (2007) Using stable isotope analysis to examine fall armyworm (Lepidoptera: Noctuidae) host strains in a cotton habitat. J Econ Entomol 100:1569–1576. https://doi.org/10.1093/jee/100.5.1569
Nagoshi RN, Meagher RL, Hay-Roe M (2012) Inferring the annual migration patterns of fall armyworm (Lepidoptera: Noctuidae) in the United States from mitochondrial haplotypes: fall armyworm migration. Ecol Evol 2:1458–1467. https://doi.org/10.1002/ece3.268
Oeh U, Dyker H, Lösel P, Hoffmann KH (2001) In vivo effects of Manduca sexta allatotropin and allatostatin on development and reproduction in the fall armyworm, Spodoptera frugiperda (Lepidoptera, Noctuidae). Invertebr Reprod Dev 39:239–247. https://doi.org/10.1080/07924259.2001.9652488
Overton K, Maino JL, Day R et al (2021) Global crop impacts, yield losses and action thresholds for fall armyworm (Spodoptera frugiperda): a review. Crop Prot 145:105641. https://doi.org/10.1016/j.cropro.2021.105641
Pair SD, Raulston JR, Sparks AN et al (1986) Fall armyworm distribution and population dynamics in the southeastern states. The Fla Entomol 69:468–487. https://doi.org/10.2307/3495380
Pashley DP, Hardy TN, Hammond AM (1995) Host effects on developmental and reproductive traits in fall armyworm strains (Lepidoptera: Noctuidae). Ann Entomol Soc Am 88:748–755. https://doi.org/10.1093/aesa/88.6.748
Pasquali S, Mariani L, Calvitti M et al (2020) Development and calibration of a model for the potential establishment and impact of Aedes albopictus in Europe. Acta Trop. https://doi.org/10.1016/j.actatropica.2019.105228
Ponti L, Gilioli G, Biondi A et al (2015) Physiologically based demographic models streamline identification and collection of data in evidence-based pest risk assessment. EPPO Bull 45:317–322. https://doi.org/10.1111/epp.12224
Rafikov M, Balthazar JM, von Bremen HF (2008) Mathematical modeling and control of population systems: Applications in biological pest control. Appl Math Comput 200:557–573. https://doi.org/10.1016/j.amc.2007.11.036
Ramasamy M, Das B, Ramesh R (2021) Predicting climate change impacts on potential worldwide distribution of fall armyworm based on CMIP6 projections. J Pest Sci. https://doi.org/10.1007/s10340-021-01411-1
Ramirez-Cabral NYZ, Kumar L, Shabani F (2017) Future climate scenarios project a decrease in the risk of fall armyworm outbreaks. J Agric Sci 155:1219–1238. https://doi.org/10.1017/S0021859617000314
Régnière J, St-Amant R, Duval P (2012a) Predicting insect distributions under climate change from physiological responses: spruce budworm as an example. Biol Invasions 14:1571–1586. https://doi.org/10.1007/s10530-010-9918-1
Régnière J, Powell J, Bentz B, Nealis V (2012b) Effects of temperature on development, survival and reproduction of insects: experimental design, data analysis and modeling. J Insect Physiol 58:634–647. https://doi.org/10.1016/j.jinsphys.2012.01.010
Ríos-Díez JD, Saldamando-Benjumea CI (2011) Susceptibility of Spodoptera frugiperda (Lepidoptera: Noctuidae) strains from central colombia to two insecticides, methomyl and lambda-cyhalothrin: a study of the genetic basis of resistance. J Econ Entomol 104:1698–1705. https://doi.org/10.1603/EC11079
Rojas JC, Virgen A, Malo EA (2004) Seasonal and nocturnal flight activity of Spodoptera frugiperda males (Lepidoptera: Noctuidae) monitored by pheromone traps in the coast of Chiapas, Mexico. Fla Entomol 87:496–503. https://doi.org/10.1653/0015-4040(2004)087[0496:SANFAO]2.0.CO;2
Rose AH, Silversides RH, Lindquist OH (1975) Migration flight by an aphid, Rhopalosiphum maidis (Hemiptera: Aphididae), and a noctuid, Spodoptera frugiperda (Lepidoptera: Noctuidae). Can Entomol 107:567–576. https://doi.org/10.4039/Ent107567-6
Rossi V, Sperandio G, Caffi T et al (2019) Critical success factors for the adoption of decision tools in IPM. Agronomy 9:710. https://doi.org/10.3390/agronomy9110710
Salas-Araiza MD, Martínez-Jaime OA, Guzmán-Mendoza R et al (2018) Fluctuación poblacional de Spodoptera frugiperda (JE Smith) y Spodoptera exigua (Hubner)(Lepidoptera: Noctuidae) mediante el uso de feromonas en Irapuato, Gto. Mex Entomol Mex 5:368–374
Salazar-Blanco JD, Cadet-Piedra E, González-Fuentes F (2020) Monitoreo de Spodoptera spp. en caña de azúcar: uso de trampas con feromonas sexuales. Agron Mesoam 31:445–459. https://doi.org/10.15517/am.v31i2.39046
Sarr OM, Garba M, Bal AB et al (2021) Strain composition and genetic diversity of the fall armyworm Spodoptera frugiperda (Lepidoptera, Noctuidae): new insights from seven countries in West Africa. Int J Trop Insect Sci 41:2695–2711. https://doi.org/10.1007/s42690-021-00450-6
Sartiami D, Dadang HI et al (2020) First record of fall armyworm (Spodoptera frugiperda) in Indonesia and its occurence in three provinces. IOP Conf Ser Earth Environ Sci 468:012021. https://doi.org/10.1088/1755-1315/468/1/012021
Schlemmer M (2018) Effect of temperature on development and reproduction of Spodoptera frugiperda (Lepidoptera: Noctuidae). Dissertation, North-West University. http://repository.nwu.ac.za/handle/10394/31294. Accessed 16 Mar 2021
Schulzweida U (2019) CDO user guide. 1–206. https://doi.org/10.5281/zenodo.2558193
Sharanabasappa CM, Kalleshwaraswamy J, Poorani MS et al (2019) Natural enemies of Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae), a recent invasive pest on maize in South India. Fla Entomol. https://doi.org/10.1653/024.102.0335
Silvain JF, Ti-A-Hing J (1985) Prediction of larval infestation in pasture grasses by Spodoptera frugiperda (Lepidoptera: Noctuidae) from estimates of adult abundance. Fla Entomol 68:686. https://doi.org/10.2307/3494875
Simmons AM (1993) Effects of Constant and fluctuating temperatures and humidities on the survival of Spodoptera frugiperda pupae (Lepidoptera: Noctuidae). Fla Entomol 76:333. https://doi.org/10.2307/3495733
Soberon J, Nakamura M (2009) Niches and distributional areas: concepts, methods, and assumptions. Proc Natl Acad Sci USA 106:19644–19650. https://doi.org/10.1073/pnas.0901637106
Solari HG, Natiello MA (2014) Linear processes in stochastic population dynamics: theory and application to insect development. Sci World J 2014:1–15. https://doi.org/10.1155/2014/873624
Sparks AN (1979) A review of the biology of the fall armyworm. Fla Entomol 62:82. https://doi.org/10.2307/3494083
Sperandio G (2021) Models supporting decision-making in pest management. The role of scales and contexts of application. Dissertation, University of Modena and Reggio Emilia
Suby SB, Soujanya PL, Yadava P et al (2020) Invasion of fall armyworm (Spodoptera frugiperda) in India: nature, distribution, management and potential impact. Curr Sci 119(1):44–51. https://doi.org/10.18520/cs/v119/i1/44-51
Suh S-J, Choi D-S, Na S (2021) Occurrence status of the fall armyworm (Lepidoptera: Noctuidae) in South Korea. Insecta Mundi
Tambo JA, Kansiime MK, Rwomushana I et al (2021) Impact of fall armyworm invasion on household income and food security in Zimbabwe. Food Energy Secur 10:299–312. https://doi.org/10.1002/fes3.281
Tepa-Yotto GT, Tonnang HEZ, Goergen G et al (2021) Global habitat suitability of Spodoptera frugiperda (JE Smith) (Lepidoptera, Noctuidae): key parasitoids considered for its biological control. Insects 12:273. https://doi.org/10.3390/insects12040273
Timilsena BP, Niassy S, Kimathi E et al (2022) Potential distribution of fall armyworm in Africa and beyond, considering climate change and irrigation patterns. Sci Rep 12:1–15. https://doi.org/10.1038/s41598-021-04369-3
Tingle FC, Mitchell ER (1979) Spodoptera frugiperda: factors affecting pheromone trap catches in corn and peanuts. Environ Entomol 8:989–992. https://doi.org/10.1093/ee/8.6.989
Van Vuuren DP, Edmonds J, Kainuma M et al (2011) The representative concentration pathways: an overview. Clim Change 109:5–31. https://doi.org/10.1007/s10584-011-0148-z
Varella AC, Menezes-Netto AC, de Alonso JD, S, et al (2015) Mortality dynamics of Spodoptera frugiperda (Lepidoptera: Noctuidae) immatures in maize. PLoS ONE 10:e0130437. https://doi.org/10.1371/journal.pone.0130437
Vilarinho EC, Fernandes OA, Hunt TE, Caixeta DF (2011) Movement of Spodoptera frugiperda adults (Lepidoptera: Noctuidae) in maize in Brazil. Fla Entomol 94:480–488. https://doi.org/10.1653/024.094.0312
Westbrook JK, Nagoshi RN, Meagher RL et al (2016) Modeling seasonal migration of fall armyworm moths. Int J Biometeorol 60:255–267. https://doi.org/10.1007/s00484-015-1022-x
Wiens JA, Stralberg D, Jongsomjit D et al (2009) Niches, models, and climate change: assessing the assumptions and uncertainties. Proc Natl Acad Sci USA 106:19729–19736. https://doi.org/10.1073/pnas.0901639106
Zacarias DA (2020) Global bioclimatic suitability for the fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae), and potential co-occurrence with major host crops under climate change scenarios. Clim Change 161:555–566. https://doi.org/10.1007/s10584-020-02722-5
Zaimi S, Saranum M, Hudin L, Ali W (2021) First incidence of the invasive fall armyworm, Spodoptera frugiperda (J.E. Smith, 1797) attacking maize in Malaysia. Bioinvasions Rec 10:81–90. https://doi.org/10.3391/bir.2021.10.1.10
Zanuncio JC, da Silva CAD, de Lima ER et al (2008) Predation rate of Spodoptera frugiperda (Lepidoptera: Noctuidae) larvae with and without defense by Podisus nigrispinus (Heteroptera: Pentatomidae). Braz Arch Biol Technol 51:121–125. https://doi.org/10.1590/S1516-89132008000100015
Zhang Z, Batuxi JY et al (2021) Effects of different wheat tissues on the population parameters of the fall armyworm (Spodoptera frugiperda). Agronomy 11:2044. https://doi.org/10.3390/agronomy11102044
Acknowledgements
We kindly thank the Editor and the anonymous Reviewers of this manuscript. Special thanks go to Dr Michele Colturato (Department of Mathematics, University of Pavia, Pavia, Italy) for his contribution to the preliminary version of the code. We kindly thank Mr Gabriele Merli (Department of Mathematics, University of Pavia, Pavia, Italy) and Professor Luca Pavarino (Department of Mathematics, University of Pavia, Pavia, Italy), for providing computing resources of the new high-performance computing cluster (HPC) of the University of Pavia. We acknowledge the Copernicus Climate Change Service for making available the ERA5-Land hourly data used in the present study. We acknowledge the World Climate Research Programme’s Working Group on Regional Climate, and the Working Group on Coupled Modelling, former coordinating CORDEX and responsible panel for CMIP5 for providing the climatic dataset used in the present study. We also acknowledge the Earth System Grid Federation infrastructure an international effort led by the U.S. Department of Energy’s Program for Climate Model Diagnosis and Intercomparison, the European Network for Earth System Modelling, and other partners in the Global Organisation for Earth System Science Portals (GOESSP).
Funding
Open access funding provided by Università degli Studi di Brescia within the CRUI-CARE Agreement. The authors did not receive support from any organisation for the submitted work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Communicated by Jon Sweeney .
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gilioli, G., Sperandio, G., Simonetto, A. et al. Assessing the risk of establishment and transient populations of Spodoptera frugiperda in Europe. J Pest Sci 96, 1523–1537 (2023). https://doi.org/10.1007/s10340-022-01517-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-022-01517-0